PR2000
submitted to the Annual Progress Report of the Society for Atomic Collision Research, 2000.
[PDF File]
ABSOLUTE YIELDS OF THE TOTAL DESORPTION FROM THE SURFACE OF SOLID ARGON BY LOW ENERGY ELECTRON IMPACT
Takashi Adachi1*, Takato Hirayama and Ichiro Arakawa
Department of Physics, Gakushuin University, Tokyo, 171-8588 JAPAN
1. Introduction
Electron or photon irradiation of a solid surface produces the electronic excitation which can be followed by the desorption of various kinds of particles. The desorption at the surface of a rare gas solid has been extensively studied for last two decades1). Investigation of this phenomenon will reveal the dynamics of the electronic excitations and the relaxation processes in the solid. As to the desorption of excited neutral particles from rare gas solids, various desorption mechanisms were proposed and some of them were confirmed experimentally and theoretically. On the other hand, the desorption mechanism of the ground state atom has not been fully understood yet, though it is the main component of the desorbed particles. In our previous work, we reported the total photo-desorption yields from the surface of solid neon at the excitonic excitation energy and revealed the main desorption channel by the quantitative analysis2). In this report, we present the results of the absolute measurements of the total desorption yields of solid argon by low energy electron bombardment. The "total" means that we detected all the argon particles desorbed, i.e., atoms and clusters in ground, electronically excited, or ionized states.
2. Experimental
The experimental set-up and procedures have been described in detail elsewhere2,3) and are briefly summarized here. A liquid helium cryostat was installed in an ultrahigh-vacuum chamber with a base pressure of 1 ¥ 10-8 Pa. Argon gas was introduced into the chamber and condensed on the surface of a Cu (111) substrate attached to the cryostat. The substrate was kept at the temperature of 6 K or lower during the experiment. The thickness of argon film was calculated from the exposure on the assumption that the condensation coefficient of argon at the temperature of 6 K was unity. The uncertainty of the thickness of the sample is estimated at ±50 %, which is mainly caused by the uncertainty of sensitivity of the gauge for argon and the condensation coefficient of argon gases.
The absolute desorption yields were calculated from the incident electron current and the number of desorbed argon atoms. The absolute amount of the desorbed argon was calculated from the pumping speed for argon and the rise of the argon partial pressure in the vacuum chamber during the electron irradiation of the sample. The pumping speed for argon of a turbo molecular pump and the cold surface of the cryostat was 0.05 ± 0.01 m3/s in total, which was determined by gas flow method and a Baratron pressure gauge which was installed in a gas handling system. The partial pressure of argon was measured by a quadrupole mass spectrometer which was calibrated with a Bayard-Alpert ionization gauge which was installed in the main chamber at each run of the experiments. The experimental uncertainty is estimated to be 50% by a quadrature sum of all sources of error.
3. Results and Discussion
Figure 1 shows the thickness dependence of the absolute desorption yield of argon by 220 eV electron impact. The desorption yield enhances as the thickness of the film increases from about 0.1 atomic layers, and has a peak at about 2 atomic layers. After passing through a minimum at about 10 atomic layers, the yield rises again and reaches to about 1.0 (atoms/electron) at about 300 atomic layers.
The desorption mechanism for thin films (<10 atomic layers) can be explained by the Antoniewicz mechanism4). The primary process in this mechanism is the ionization of an atom on the metal substrate. An adparticle ionized by the incident electron moves towards the substrate due to an attractive image force and can be neutralized via electron tunneling from the substrate. If the kinetic energy of the neutralized atom is larger than the binding energy at the neutralized position, the neutral atom can desorb after bouncing from the substrate. The desorption via this mechanism is dominant for a monolayer film because in a thicker film the overlayer atoms prevent the desorption of the neutralized atom. The present experimental results, however, shows a maximum at about 2 atomic layers. This discrepancy may be due to the error in determining the thickness of the sample film and/or the inhomogeneous thickness of the sample.
The collision cascade model by Cui et al.5) is used to describe the desorption mechanism for the thick sample (> 300 atomic layers) quantitatively. They calculated using a classical molecular dynamics the desorption yields versus depth of excitations for solid argon in which an atom was started at a given depth in the solid with a kinetic energy in the range 0.3-3.0 eV and with a random initial direction of the motion. To estimate the total desorption yields from their calculated results, we require three parameters, the number of the excitations, the kinetic energy of the energetic atoms created in the bulk, and the desorption yield by the excitation of the surface atoms. We need the third parameter because the calculation by Cui et al. does not include the contribution of the energetic atoms created in the surface layer.
The number of the excitations in the argon film is estimated using ionization cross section in gas phase6) because the excitation cross section of 3p54s state7) is about 10 % of ionization cross section at 220 eV electron energy. The number of created ions by an electron of a kinetic energy of 220 eV is estimated at 0.17 ions/electron in the surface layer. In the n-th underlying layer, a 220 eV electron creates 0.17 ¥ (1-0.17)n ions. The number of ions created by the electron of 220 eV as a function of the layer number is shown by filled circles in fig.2. If the scattered electron has an energy larger than the ionization energy of argon, it can create an additional ion. The number of ions created by the electrons which are scattered once to four times are also shown in fig.2. Here, we used the value of the electron mean energy loss W of 24 eV in the ionization event which is reported for the electron of 0.5 - 3 keV in liquid phase8). Because the mean energy loss W is small compared to the initial electron energy, we assume that the scattered electron has a sharp angular distribution along the direction of the incidence. We ignore the contribution of ejected electrons because they do not have enough energy to cause the ionization of argon. As Cui's calculation shows that the energetic atoms created deeper than the 6th atomic layer do not contribute to the desorption, we only take account of ions which are created between the 1st and 6th atomic layers from the surface of solid argon. The total number of created ions in each layer is shown as crosses in fig.2.
The kinetic energy of 0.4 eV is used in our estimation for the energetic atoms created in the bulk. This is the energy of atoms desorbed from the surface of argon solid via the dissociation of a vibrationally relaxed excimer9). We assume that every created ion produces two energetic atoms in the following way: The ion forms an ionic dimer which becomes an excited dimer (excimer) via electron-hole recombination process. The excimer dissociates after the vibrational relaxation producing two energetic atoms with kinetic energies of 0.4 eV9)
We estimated the desorption yield by the excitation of the surface atoms from the desorption probability at the surface (0.3 atoms/excitation) which is obtained from the selective excitation of the surface excitons by photon stimulated desorption experiments10).
With these parameters, we finally obtain the desorption yield of 0.4 atoms/electron for 220eV electron bombardment. This value is about half of our experimental result. Better agreement is expected if we consider the diffusion of the excitation in the solid which is neglected in our estimation.
4. Summary
We have measured the total desorption yield from the surface of solid argon by 220 eV electron bombardment. It is found that the main desorption mechanism is the collision cascade initiated by a creation of energetic atoms in the solid which are produced via the dissociation of a vibrationally relaxed excimer in the solid. The quantitative evaluation of the absolute desorption yield which takes the diffusion of the excitation into account is now in progress.
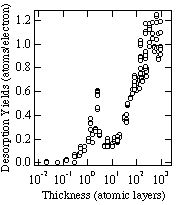
Fig.1 Thickness dependence of the total desorption yields for solid argon measured at the incident electron energy of 220 eV.
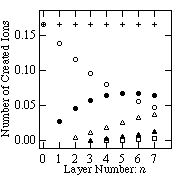
Fig.2 The total number of created ions for 220 eV incident electron (crosses). Also shown are the number of ions created by the incident electrons (open circles, Ee = 220 eV), and by electrons scattered once (solid circles, Ee = 196 eV), two times (open triangles, Ee = 172 eV), three times (solid triangles, Ee = 148 eV), and four times (rectangles, Ee = 124 eV). Note n = 0 for the surface layer. See text for detail.
REFERENCES
1) for recent review, see I. Arakawa, Molec. Cryst. Liq. Cryst, 314, 47 (1998), M. Runne and G. Zimmerer, Nucl. Instr. Meth. Phys. Res., B101, 156 (1995).
2) I. Arakawa, T. Adachi, T. Hirayama and M. Sakurai, Surf. Sci. 451, 136 (2000).
3) M. Sakurai, T. Hirayama, and I. Arakawa, Vacuum, 41, 217 (1990).
4) P. R. Antoniewicz, Phys. Rev., B21, 3811 (1980).
5) S. Cui, R. E. Johnson, and P. Cummings, Surf. Sci., 207, 186 (1988).
6) H. C. Straub, P. Renault, B. G. Lindsay, K. A. Smith, and R. F. Stebbings, Phys. Rev., A 52, 1115 (1995).
7) S. Tsurubuchi, T. Miyazaki and K. Motohashi, J. Phys., B 9, 1785 (1996).
8) M. Miyajima T. Takahashi, S. Konno, T. Hamada, S. Kubota, K. Shibamura and T. Doke, Phys. Rev., A 9, 1438 (1974).
9) E. Hudel, E. Steinacker, and P. Feulner, Phys. Rev., B 44, 8972 (1991).
10) T. Adachi, T. Hirayama, I. Arakawa, and M. Sakurai, in preparation.
1* Fax: +81-3-3987-6732, e-mail: xchada@gakushuin.ac.jp

