Fluorescence photoswitching of a diarylethene with single-wavelength visible light
R. Kashihara, M. Morimoto, S. Ito, H. Miyasaka, M. Irie
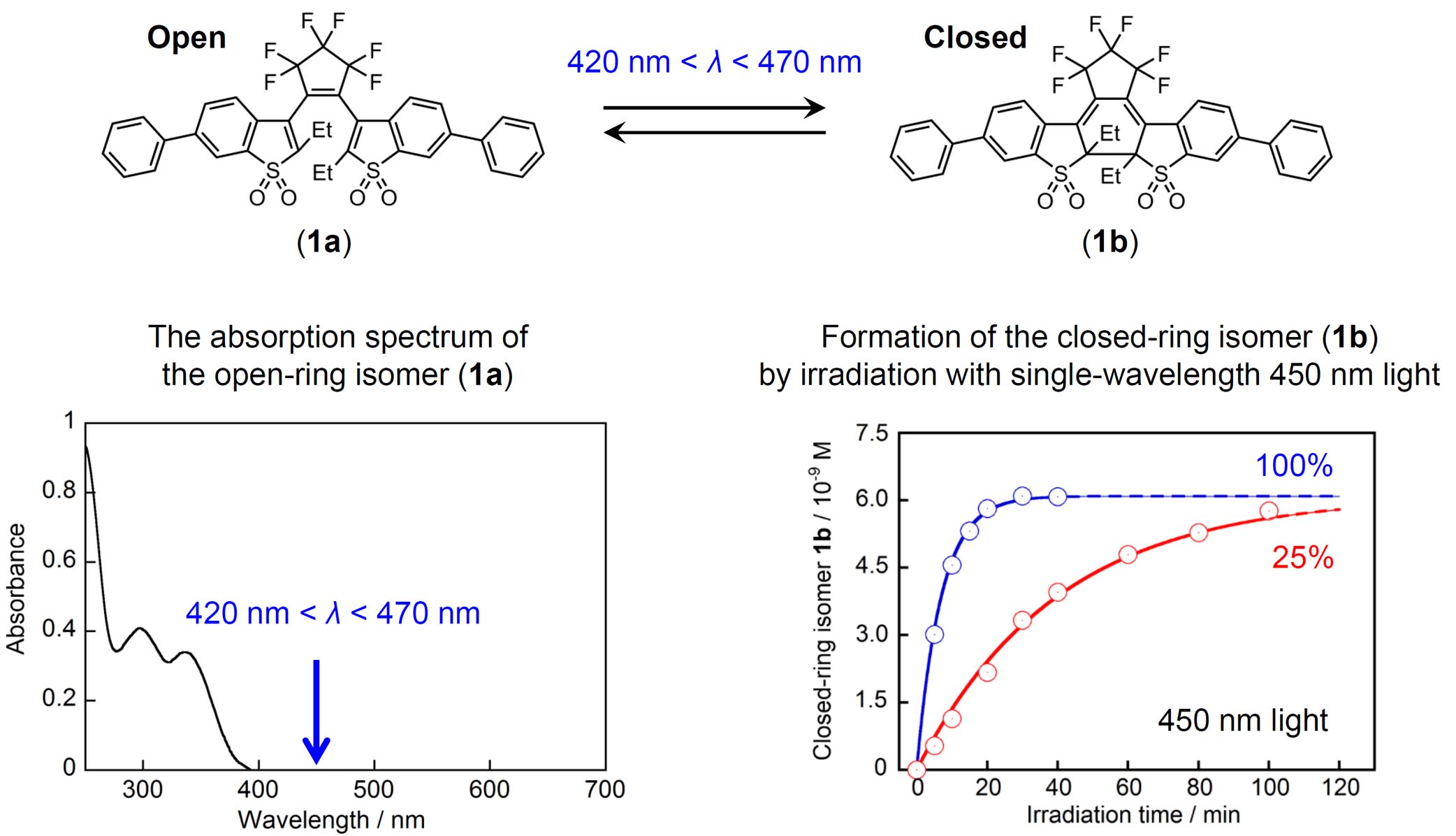
Photoswitchable turn-on mode fluorescent molecules have been so far successfully used in super-resolution fluorescence microscopies. Here, we report on fluorescence photoswitching of 1,2-bis(2-ethyl-6-phenyl-1-benzothiophene-1,1-dioxide-3-yl)perfluorocyclopentene (1) by irradiation with single-wavelength visible (420 nm < λ < 470
nm) light, the wavelength of which is longer than the 0–0 transition of
open-ring isomer 1a, without UV light excitation. By absorbing very weak hot bands or Urbach tails 1a underwent a cyclization reaction to produce fluorescent closed-ring isomer
1b. Both cyclization and cycloreversion reactions of 1 took place with the visible light in the far off-resonance region of the absorption edge. Based on numerical simulations of the formation process of 1b from 1a by irradiation with 450 nm light, weak absorption coefficients at 450
nm in n-hexane and CCl4 were estimated to be 0.084 and 0.19 M–1 cm–1, respectively. The reversible fluorescence photoswitching with the single visible light is advantageously applicable to super-resolution fluorescence imaging in biological systems
J. Am. Chem. Soc., 139, 16498-16501 (2017)

