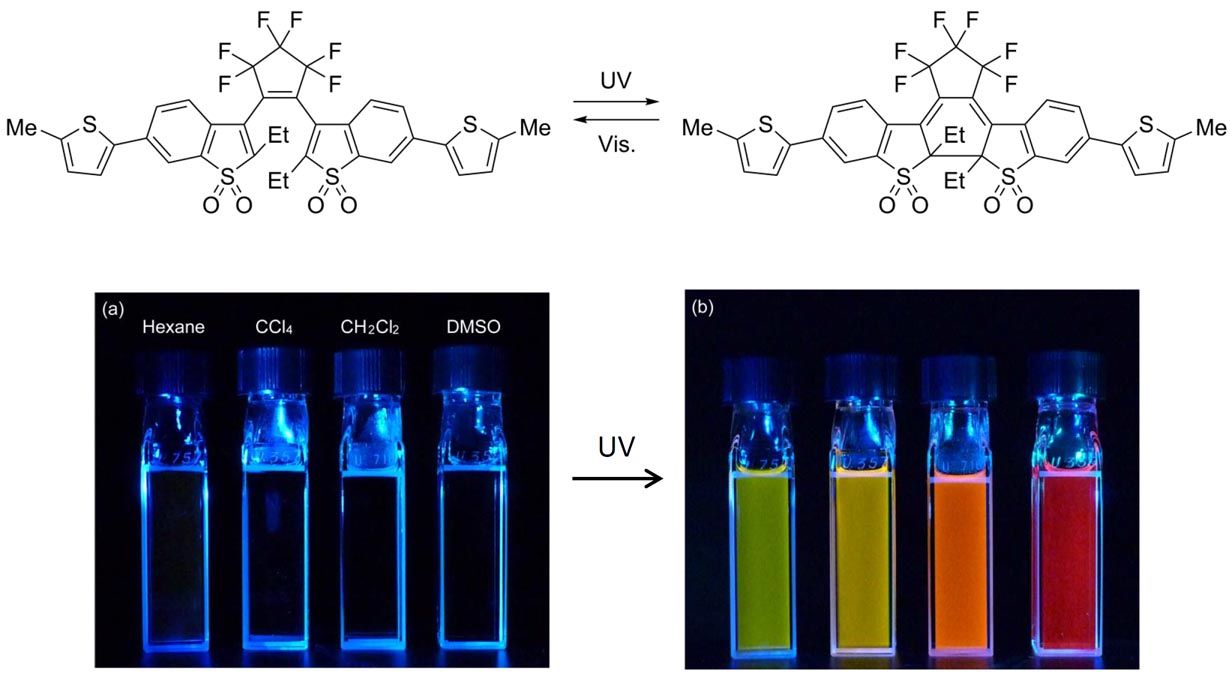
A turn-on mode fluorescent diarylethene: Solvatochromism of fluorescence
M. Morimoto, Y. Takagi, K. Hioki, T. Nagasaka, H. Sotome, S. Ito, H. Miyasaka,
M. Irie
Dyes and Pigments, 153, 144-149 (2018)
Environment-sensitive fluorophores have been used as molecular probes to
acquire physical and chemical information in microstructures. Here, we
report on solvatochromism of a fluorescent diarylethene, which is switched
by photoirradiation. A turn-on mode photoswitchable fluorescent diarylethene
derivative, 1,2-bis(2-ethyl-6-(5-methylthiophen- 2-yl)-1-benzothiophen-1,1-dioxide-3-yl)perfluorocyclopentene
(1a), underwent a cyclization reaction upon irradiation with ultraviolet (UV)
light to form fluorescent closed-ring isomer 1b. 1b dramatically changed the fluorescent color in response to solvent polarity, such as green in n-hexane, yellow in tetrachloromethane (CCl4), orange in dichloromethane (CH2Cl2), and red in dimethyl sulfoxide (DMSO), and showed high fluorescence quantum
yields of 0.6–0.8 in these solvents. The fluorescent solvatochromism is
ascribed to the intramolecular charge-transfer (ICT) character of 1b, which consists of electron-donating thiophene rings at both ends and
central electron-accepting benzothiophene 1,1-dioxide groups. The analysis
based on the Mataga-Lippert plot revealed that the difference in the dipole
moments between the excited and ground states of 1b is as large as 12 D. Such a solvatochromic fluorophore with photoswitching
ability can find potential applications in super-resolution fluorescence
imaging of microscopic polarity in biological cells and materials.